39+ S Orbital Diagram
Web What is the orbital diagram for Sulfur S. Web Orbital Diagram of All Elements Diagrams.
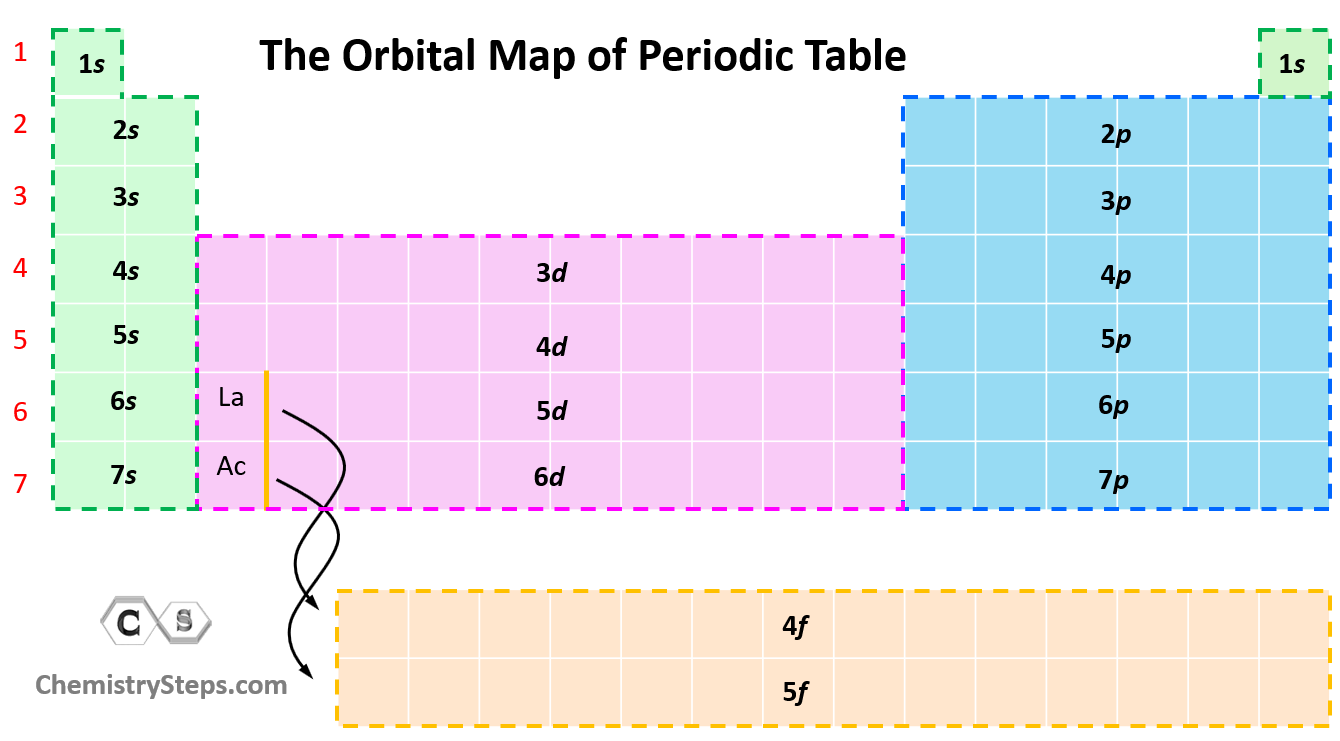
Orbital Diagrams Chemistry Steps
The shape of the orbital depends on the.
. Web In any atom with two or more electrons the repulsion between the electrons makes energies of subshells with different values of l differ so that the energy of the orbitals increases. An atomic orbital is a three-dimensional description of the location of an electron around the nucleus of an atom. The d orbital can hold 10.
Web The boundary surface diagram for the s orbital looks like a sphere having the nucleus as its centre which in two dimensions can be seen as a circle. Web This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram.
The shapes of these orbitals are discussed below. Web What is an Orbital diagram. The Sulfur orbital diagram.
Below are representations of the 3s orbital and the 3p orbitals. Web This problem has been solved. The orbital diagram for Sulfur is drawn with 5 orbitals.
The p orbital can hold 6. Youll get a detailed solution from a subject matter expert that helps you learn core concepts. Web This section also introduces the fourth quantum number an outcome of electrons possessing the property of spin.
Web According to Hunds rule as electrons are added to a set of orbitals of equal energy one electron enters each orbital before any orbital receives a second electron. Which of the orbital diagrams. Web The first two are familiar the s orbital and p orbitals.
Web The four chemically important types of atomic orbital correspond to values of ℓ 0 1 2 and 3. Web The s orbital holds a maximum of 2 electrons. Web The s orbital is a spherically-shaped region describing where an electron can be found within a certain degree of probability.
Orbitals with ℓ 0 are s orbitals and are spherically symmetrical with the greatest. But the orbitals overlap. Web These are s p d and f.
Web The first number is the principal quantum number n and the letter represents the value of l angular momentum quantum number. The orbitals are 1s 2s 2p 3s and 3p. Hence we can say that s.
1 s 2 p 3 d and 4. The third the d orbital is discussed later. The f orbital can hold 14 electrons.
This section includes worked examples sample. The s-orbitals are solid spherical shape around the nucleus.
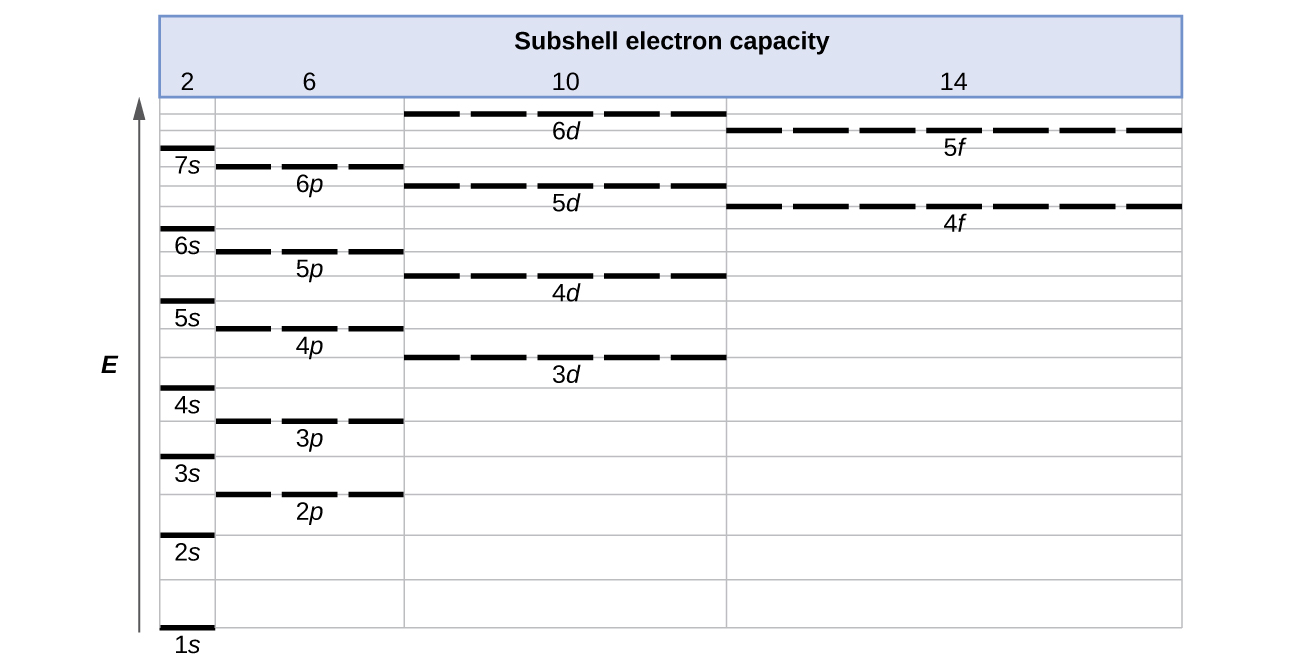
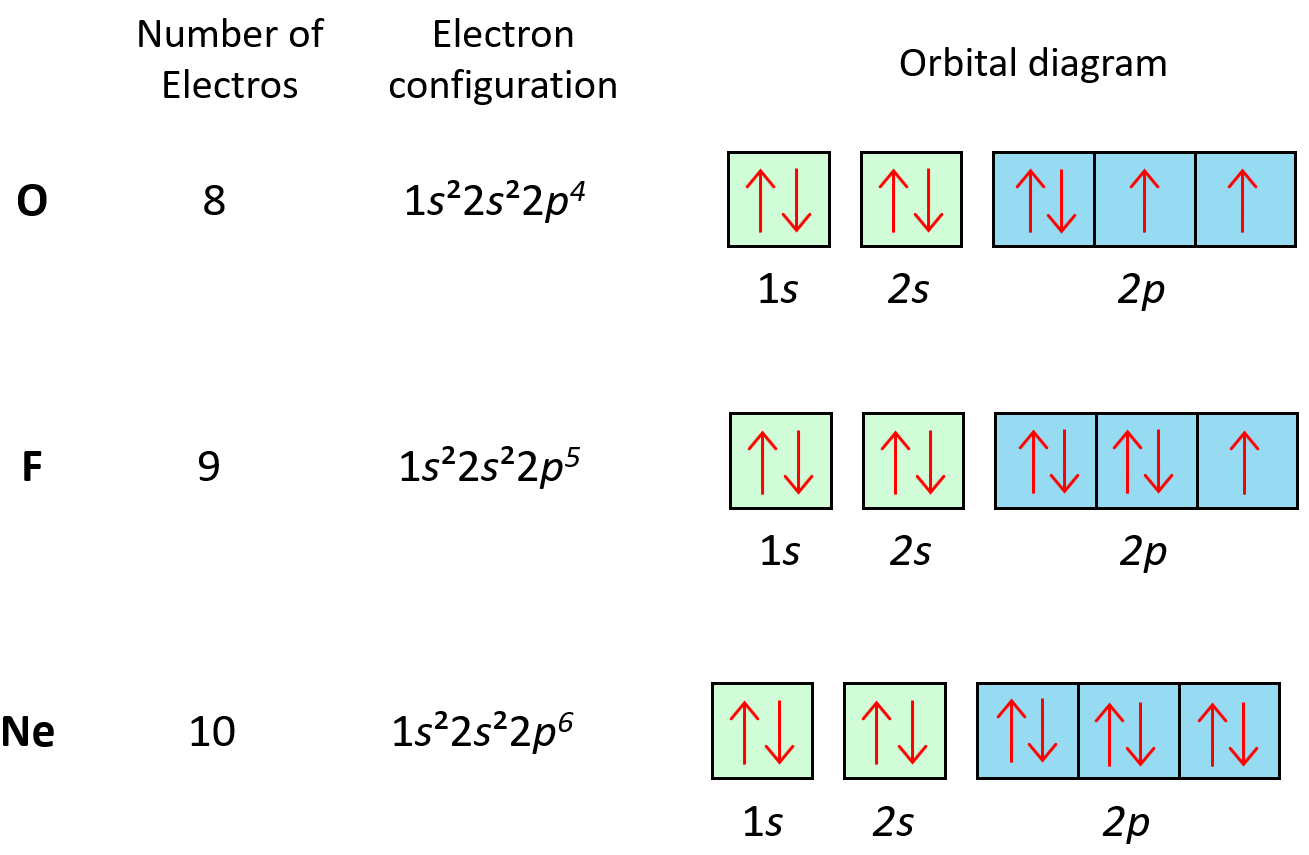
Electron Configurations Orbital Box Notation M7q7 Uw Madison Chemistry 103 104 Resource Book

Solved Which Is The Highest Occupied Molecular Orbital S Chegg Com

Orbital Diagrams Overview Examples Expii
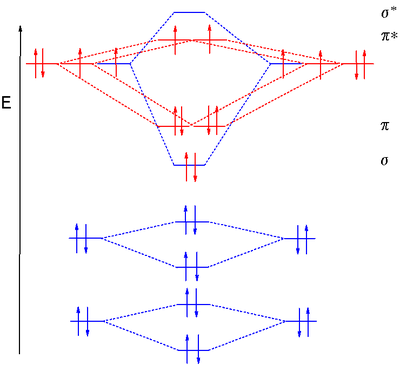
Molecular Orbital Diagram Wikipedia

Diagram Of Molecular Orbitals For Ag 17 And Ag 19 Clusters Download Scientific Diagram

Orbital Diagrams Overview Examples Expii

Orbital Diagrams And Electron Configuration Basic Introduction Chemistry Practice Problems Youtube
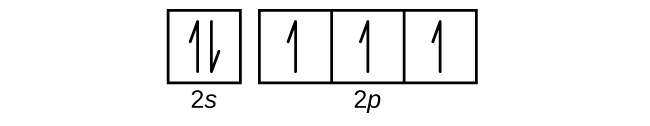
Electron Configurations Orbital Box Notation M7q7 Uw Madison Chemistry 103 104 Resource Book

How To Write The Orbital Diagram For Sulfur S Youtube
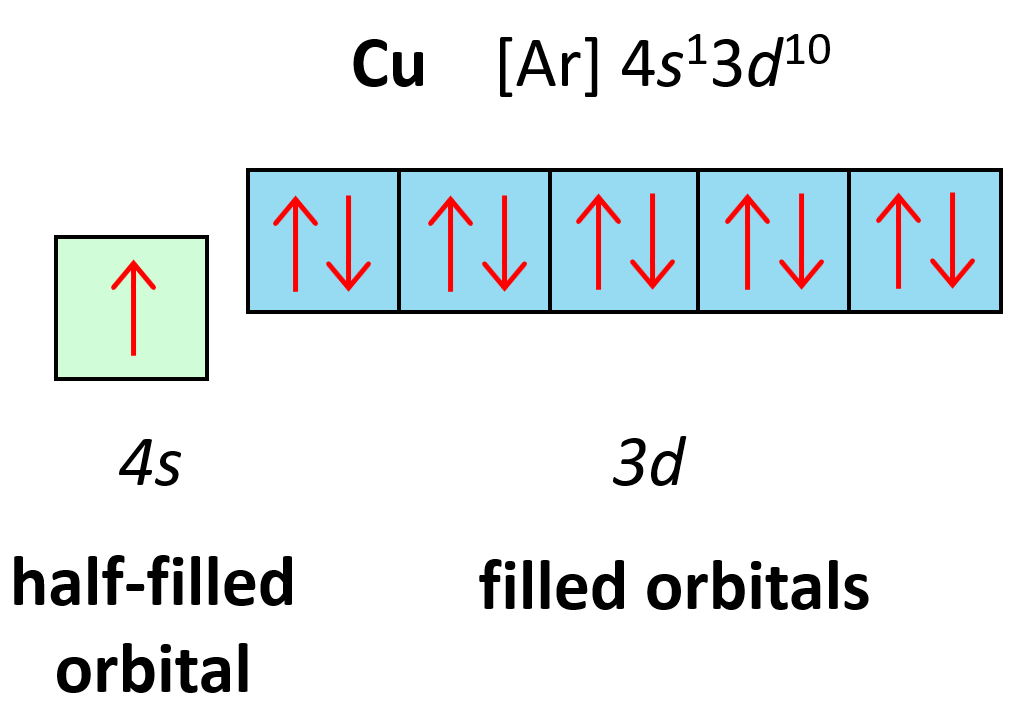
Orbital Diagrams Chemistry Steps

A Draw The Molecular Orbital Diagram For Se 2 2 B Write The Molecular Orbital Configuration For Se 2 2 C Is The Molecule Paramagnetic Or Diamagnetic D What Is The Bond Order E Is The

12 1 5 Draw The Shape Of An S Orbital And The Shapes Of The P X P Y And P Z Orbitals Youtube
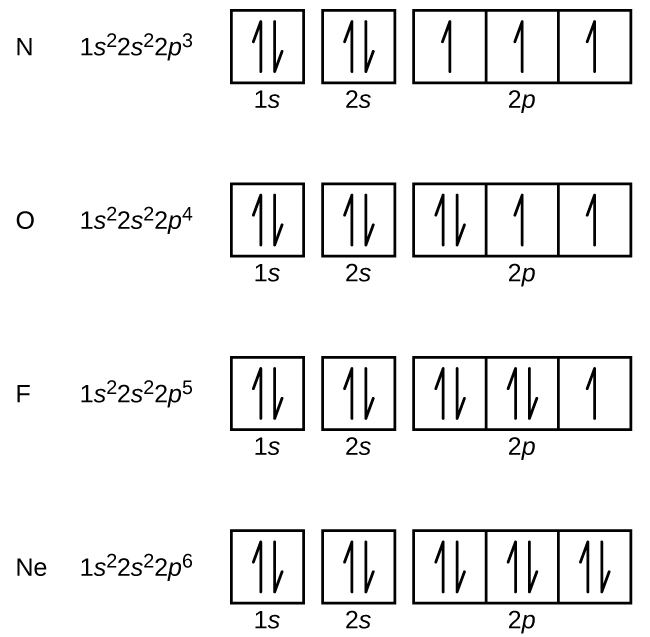
Electron Configurations Orbital Box Notation M7q7 Uw Madison Chemistry 103 104 Resource Book
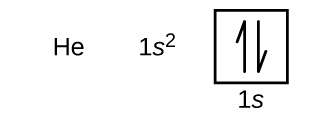
Electron Configurations Orbital Box Notation M7q7 Uw Madison Chemistry 103 104 Resource Book
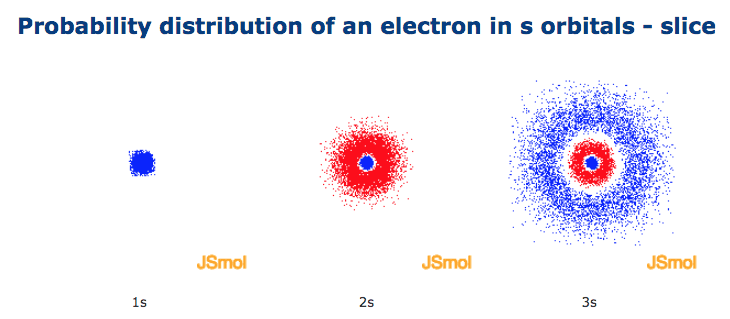
Shape Of S Orbitals In 3d

Energies Of The Occupied And Vacant Single Particle Orbitals Of W 19 Download Scientific Diagram

The Orbital Resolved Density Of States For The Pt Interface Layer In Co Download Scientific Diagram